You've probably heard a lot about the "Chinese peptides" flooding Silicon Valley—tech workers mixing powders in their kitchens, injecting themselves with insulin syringes from Amazon, attending "peptide raves" with mix-your-own workshops.
But before you go hunting for your own peptide stack, take a read through this longevity physician's guide for intelligently navigating the grey market.
This is a breakdown of what "Research Use Only" actually signals, why a 99% purity certificate doesn't mean what you think, and what each delivery route actually does to your risk profile.
1. The Appeal: Why People Take These Risks
Why are otherwise intelligent people ordering research chemicals from overseas and injecting themselves?
Because for many, the traditional system offers no solution.
- For the athlete with a torn rotator cuff, standard care is "rest and ibuprofen." BPC-157 offers the promise of active tissue repair.
- For the patient with chronic fatigue, medicine offers stimulants or dismissal. Peptides offer hope.
- For the person who can't access or afford $1,300/month Ozempic, grey-market semaglutide offers what seems to be the same molecule at one-fifth the cost.
The desire for autonomy over your own health is valid. And most people who use grey-market peptides don't end up in the ER—which is partly why the practice persists.
But survivorship bias isn't safety data. The goal of this guide isn't to stop you. It's to ensure you understand what the label on the bottle actually means—and what it doesn't.
2. The Legal Fiction: What "Research Use Only" Actually Means
When you see RUO on a vial, the vendor is making a specific legal claim: You are a researcher. This is a reagent. We are not liable if you inject it.
This is how they bypass the FDA. If they marketed these compounds for human use, they'd be shut down immediately. The RUO label is the loophole.
The Safety Gap:
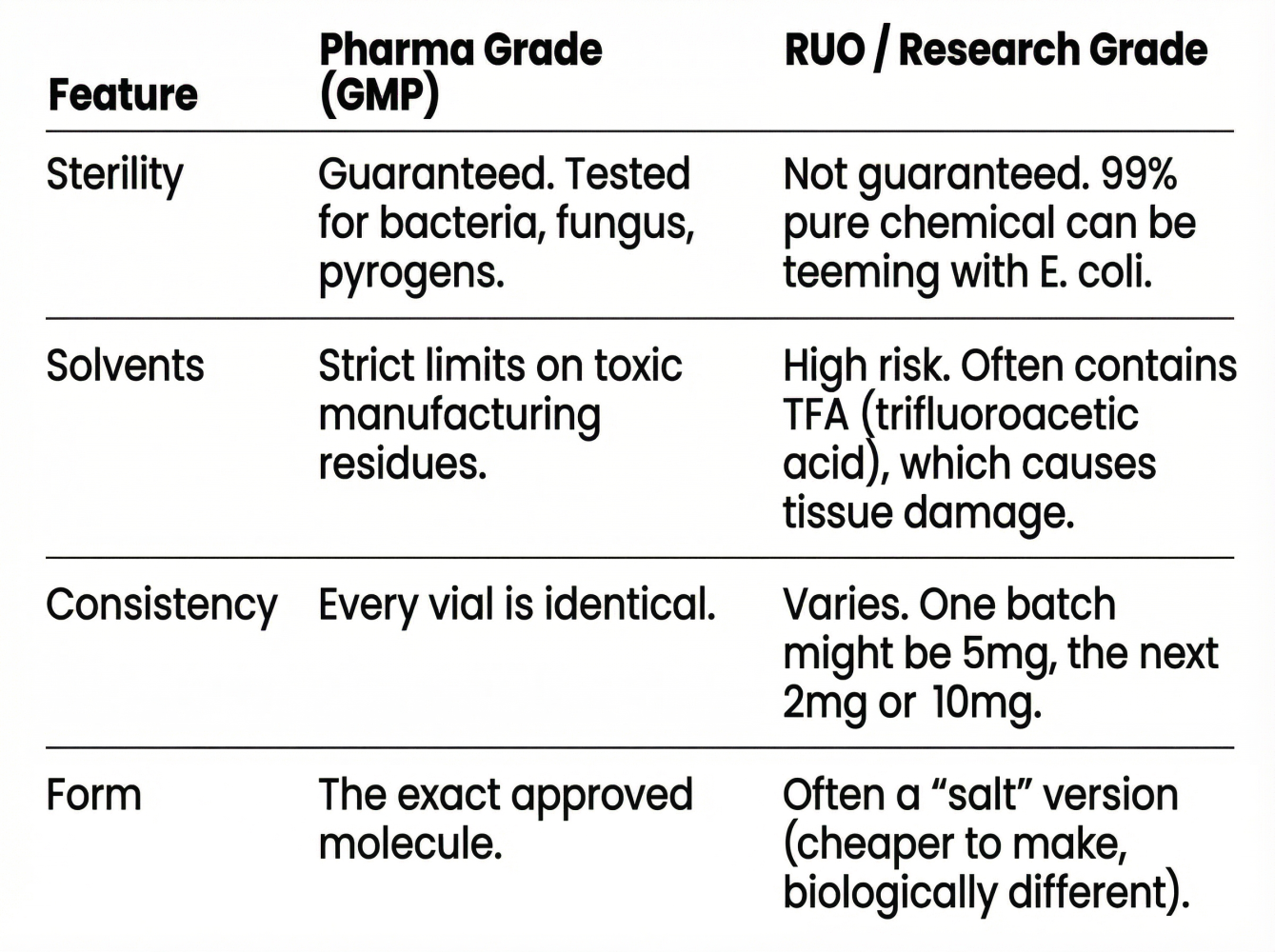
The Core Distinction: A pharmaceutical injectable has to prove it won't harm you. An RUO chemical just has to be the molecule it claims to be.
3. The Purity Trap: Why 99% Doesn't Mean Safe
When the biohacker community sends vials to Janoshik or Chromate for third-party testing, they get two pieces of information: identity (is this semaglutide?) and purity (what percentage is the target molecule?).
A 99.5% result feels reassuring. But it isn't the full picture.
What purity testing tells you:
- The chemical structure matches what you ordered
- You didn't get scammed with an inert substance
What purity testing does NOT tell you:

The "Sacrificial Vial" Problem: Testing destroys the vial. So you test Vial #1 and assume Vials #2-10 are identical. In a GMP facility with mixing standards, this assumption holds. In an unregulated lab, it's much more of a gamble.
The Dosing Problem: The semaglutide cases flooding poison control aren't primarily from bad batches—they're from math errors. Without a pre-filled pen with a lockout mechanism or clear prescription instructions, users confuse "units" (on an insulin syringe) with "milligrams," accidentally injecting 10-50x the intended dose. In the grey market, you are essentially acting as the doctor and the pharmacist, and the margin for error is zero.
4. The "Maximum Safety Protocol" (And Its Limits)
Sophisticated users follow a protocol: third-party testing, 0.22-micron syringe filters, bacteriostatic water, frozen storage.
This meaningfully reduces risk. It doesn't eliminate it.
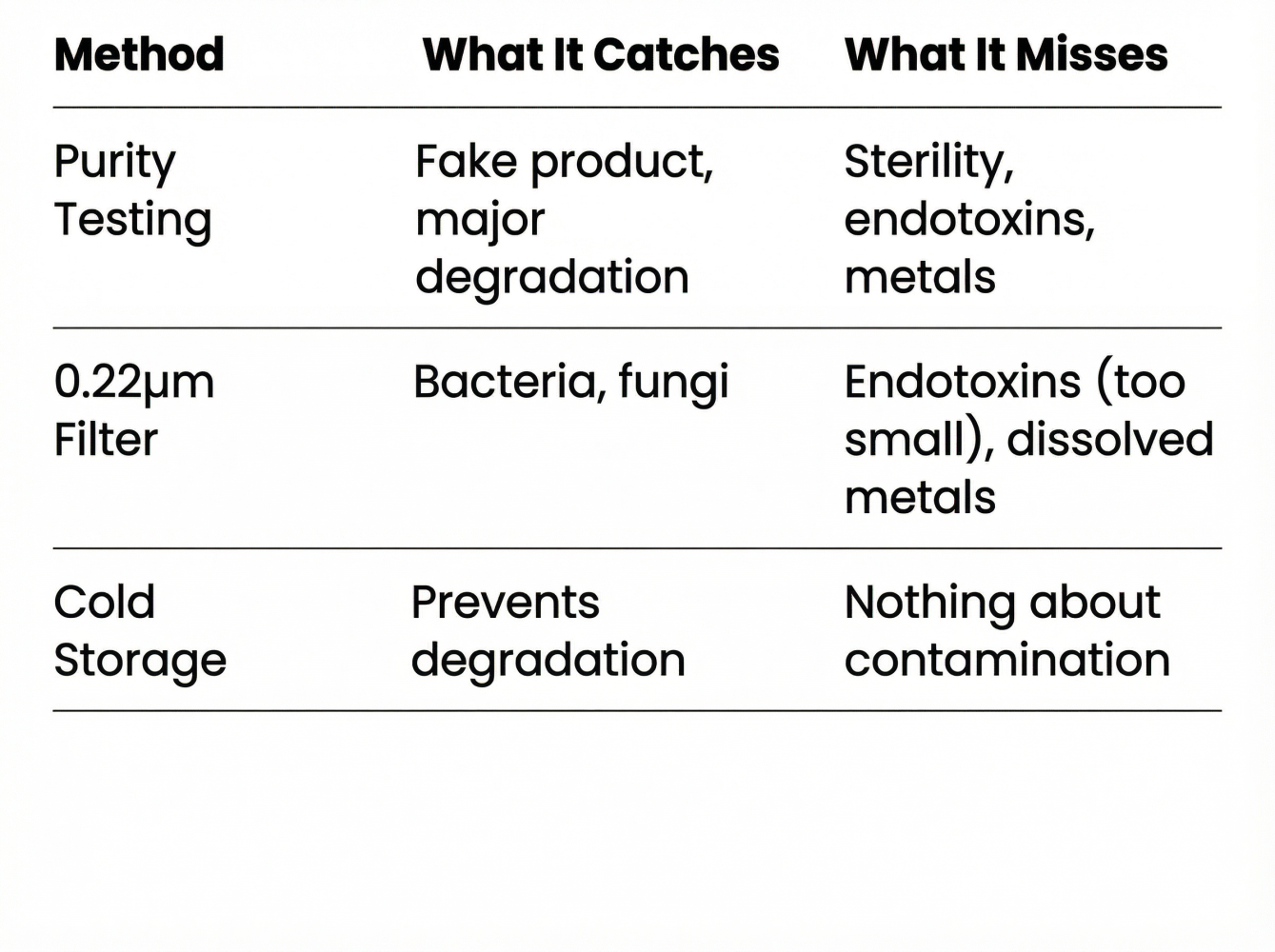
The Reality: With rigorous protocols (~$400+ on testing per batch, syringe filters, sterile technique), you can eliminate the risk of injecting fake drugs or live bacteria. You cannot fully eliminate chemical toxicity or batch-to-batch variability. You're closing most of the safety gap—but not all of it.With amazing reported results, many continue to take the risk of unregulated but accessible peptides
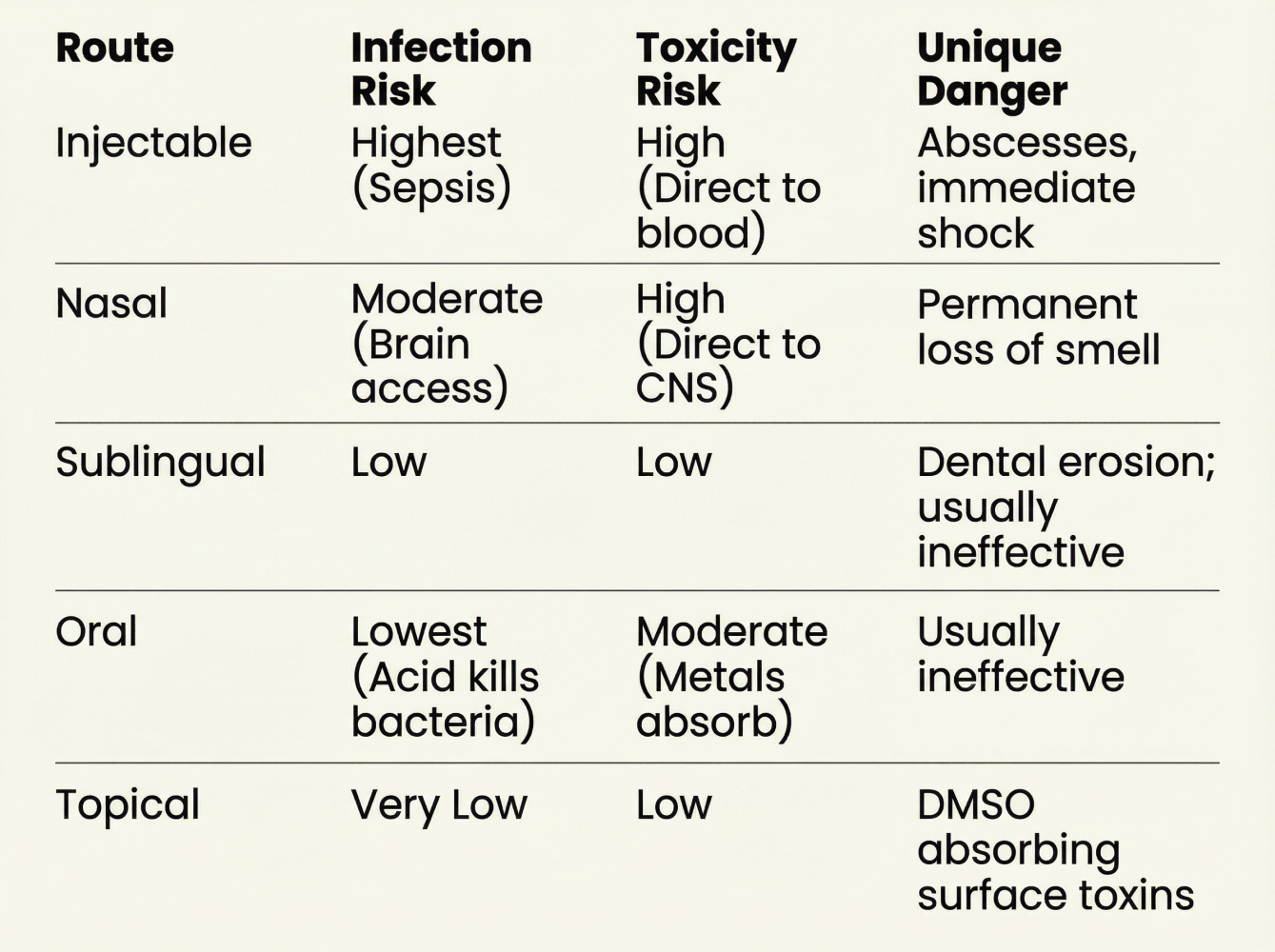
5. Delivery Routes: The Risk Hierarchy
Switching to non-injectable routes changes the safety profile—but the risks are just different.
Injectable (Highest Risk)
The grey market's most dangerous route. Direct bloodstream access means contamination = immediate systemic harm.
Documented emergencies: Abscesses requiring surgical drainage. Sepsis. Dosing errors (10-50x intended dose) causing intractable vomiting, pancreatitis. And what doesn't get talked about enough: Immunogenic reactions where antibodies attack both the drug and your natural hormones.
Nasal Sprays (Moderate-High Risk)
Common peptides: Semax, Selank, Oxytocin, PT-141
Many users assume sprays are safer (they are considered "non-sterile" compounds when made in pharmacies). This isn't always true.
The Danger: The cribriform plate (porous bone behind your nose) provides near-direct access to your brain. Contaminated nasal sprays can cause CNS infections.
Unique Risks:
- Anosmia: Wrong pH or preservatives can permanently damage olfactory mucosa (loss of smell)
- Neuro-inflammation: Impurities get "fast-track" access to your CNS
Sublingual (Moderate Risk)
Common peptides: BPC-157, Oxytocin
Safer with regards to acute emergencies, but with a major efficacy problem.
The Safety: Your mouth is already full of bacteria. Sublingual mucosa is evolved to handle non-sterile environments. You won't get sepsis from a sublingual drop.
The Hidden Risk: Holding acidic peptide solutions under your tongue daily = acid bath for your lower teeth. Long-term users report enamel erosion and gum recession.
The Efficacy Problem (The "Dalton Limit"):
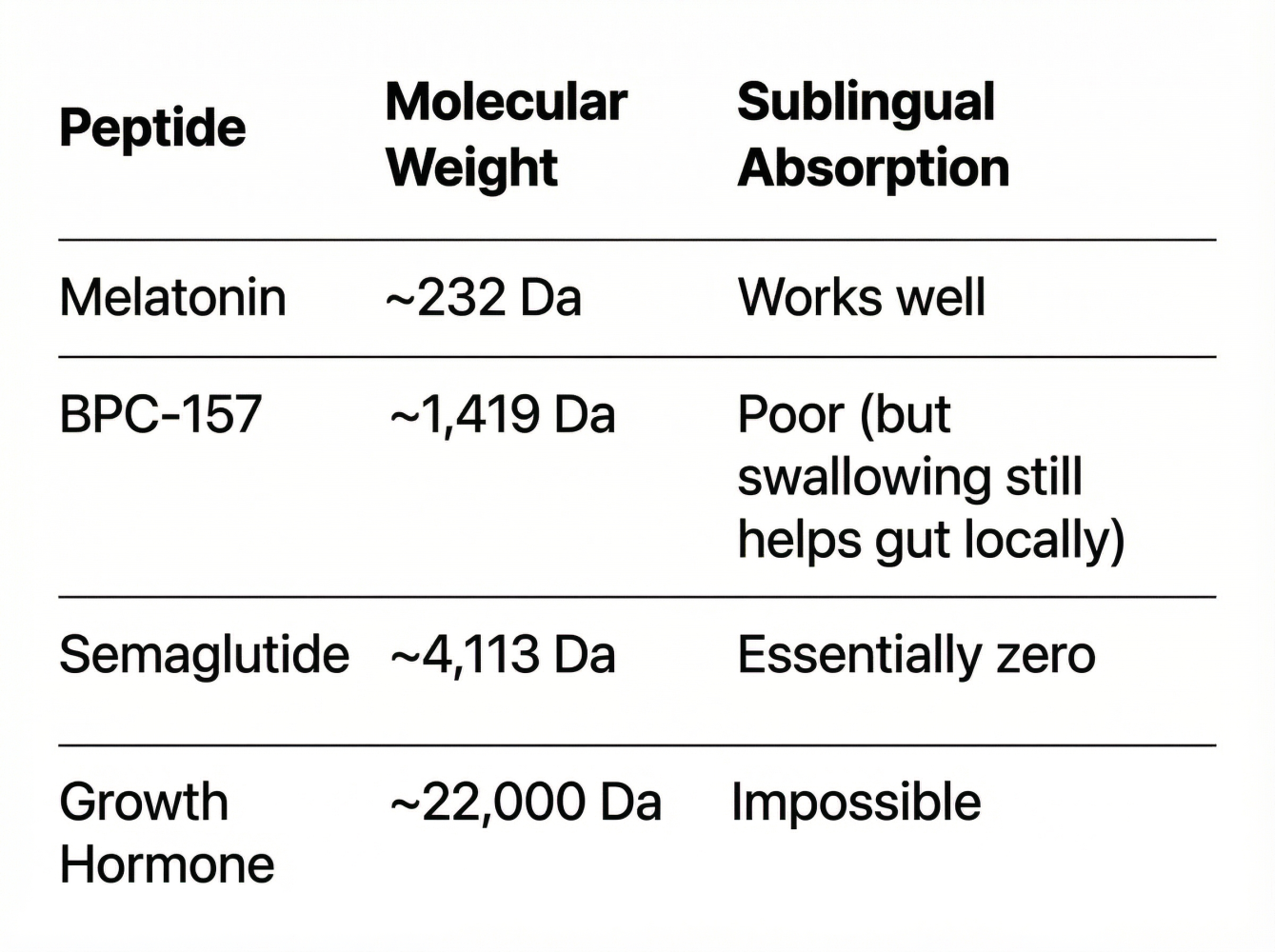
Most peptides are too large to absorb sublingually. You're essentially paying for an expensive oral dose.
Note: There is ongoing innovation in this space to create specifically formulated sublingual peptide compounds that are theoretically better absorbed.
Oral Capsules (Lowest Risk, Lowest Efficacy)
Common peptides: BPC-157 arginate, KPV, 5-Amino-1MQ
The Safety: Stomach acid (pH 1.5-3.5) is an efficient sterilization tank. It kills bacteria and denatures endotoxins. You won't get sepsis from a pill.
The Catch: Stomach acid also destroys most peptides before absorption. Unless specifically formulated (like Rybelsus with SNAC technology), you're paying for expensive urine.
The Metal Problem: Your stomach kills bacteria but absorbs heavy metals. If the powder is contaminated with lead or palladium, oral delivery doesn't protect you—it just poisons you more slowly.
Topical (Lowest Systemic Risk)
Common peptides: GHK-Cu, BPC-157
The Safety: Healthy skin is waterproof and bacteria-proof. You won't get systemic infection from a cream.
The DMSO Danger: Many peptides are too large to penetrate skin. Users add DMSO (dimethyl sulfoxide) to force absorption. DMSO drags everything through—including soap residue, dirt, or clothing dye on your skin.
Summary: Route vs. Risk
6. The Compounding Pharmacy Question
This is where confusion runs highest. Compounding pharmacies and grey-market vendors look similar online. They are not the same.
503A/503B Compounding Pharmacy:
- Licensed, inspected, regulated by the FDA
- Requires prescription
- Uses pharmaceutical-grade ingredients
- Follows USP sterility standards
- Carries malpractice insurance
Grey-Market "Research Chemical" Website:
- No prescription requirement
- No inspections
- No accountability
- "Not for Human Consumption" is their legal shield
The Category 2 Problem: In late 2023/2024, the FDA moved BPC-157, Thymosin Beta-4, and other popular peptides to Category 2 ("Substances with Safety Concerns"). Legitimate pharmacies can no longer compound them.
If a "pharmacy" is still selling BPC-157 injections today:
- They're ignoring FDA regulations—which means they're likely ignoring safety regulations too
- They're not actually a pharmacy—just a grey-market site with a medical-sounding name
When Compounding IS Safer: For GLP-1s (semaglutide/tirzepatide), sermorelin, and other peptides FDA-approved in their manufacturer form, licensed 503A/503B pharmacies are meaningfully safer than grey-market sources. Sterile clean rooms. Batch testing. State board oversight. This is worth the price difference.
7. Specific Substance Considerations
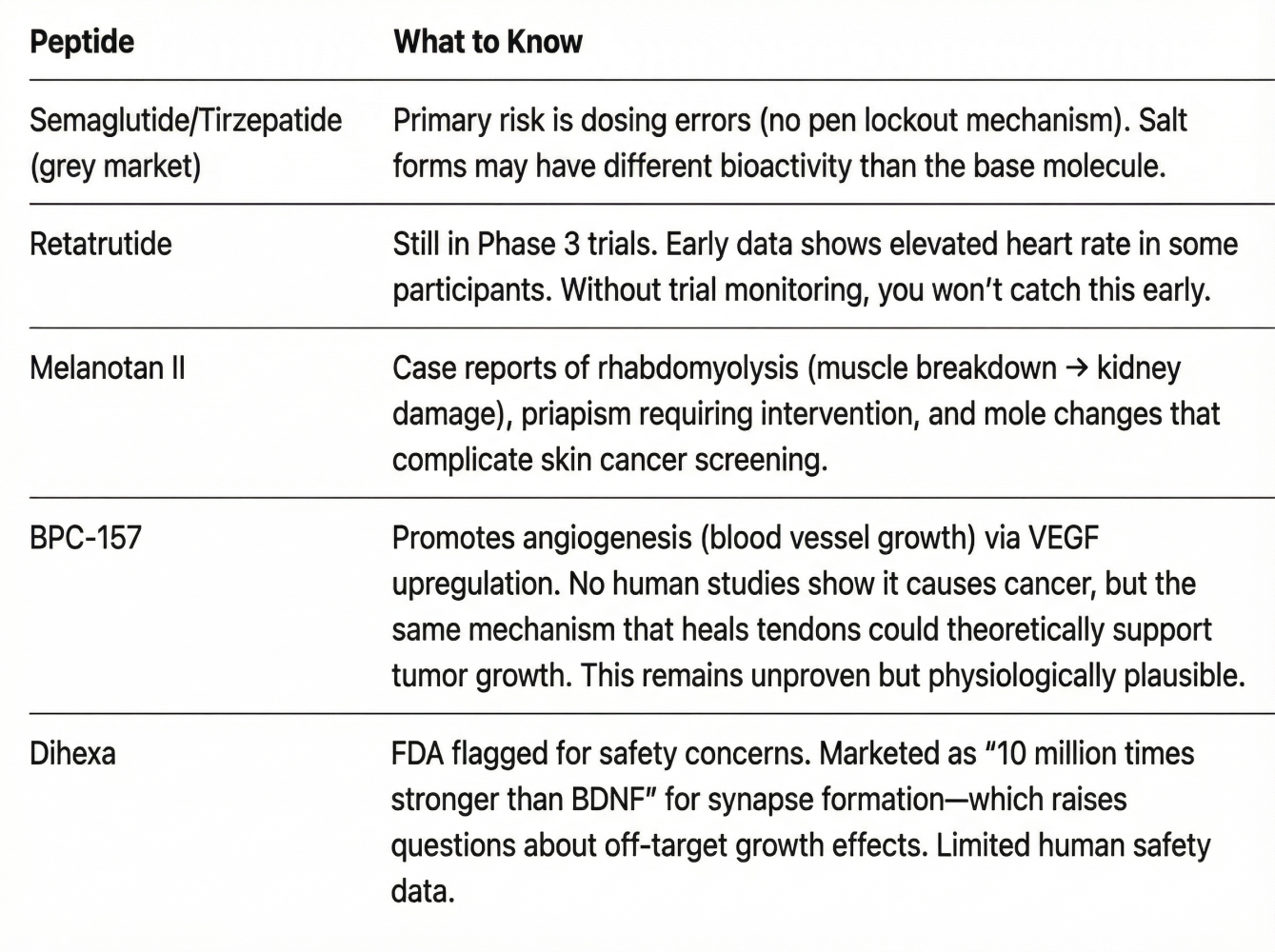
8. The Risk-Benefit Calculus
Every source exists on a spectrum. Here's how to think about the trade-offs:
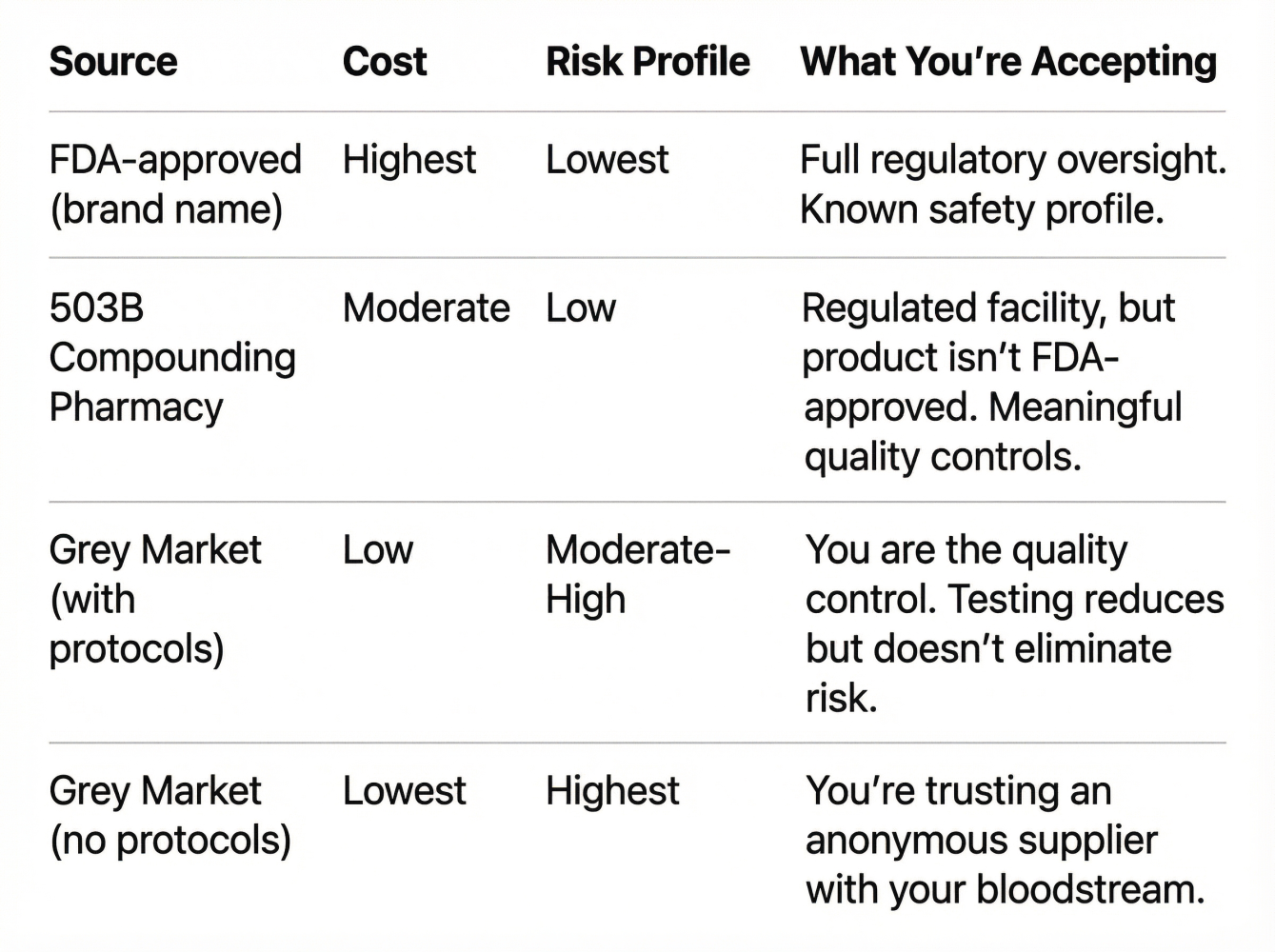
The Core Trade-off:
- Pharma grade = High cost, low risk, full accountability
- Grey market = Low cost, unknown risk, zero accountability
If you choose the grey market, you are effectively self-enrolling in an unmonitored safety trial. That's not necessarily irrational—early adopters accept risk for access. But it should be a conscious choice, not an accident of not understanding the label.
The Bottom Line
The grey market exists because of a gap in the system. Pharma companies don't fund trials for compounds that are easy to manufacture and don't target specific diseases. The FDA's approval process is too slow for an eager audience. When regulators moved BPC-157 to Category 2, they eliminated the legitimate compounding option without eliminating demand.
The people exploring grey-market peptides aren't reckless. Many are informed, systematic, and genuinely underserved by conventional medicine.
But information asymmetry is real. A purity certificate doesn't tell you if a vial is sterile. A 0.22-micron filter doesn't catch endotoxins. And "Research Use Only" is a legal classification that shifts all liability to you.
If you're going to experiment, do it with open eyes—understanding what the labels mean, what the tests catch, and what risks remain even with the best protocols.
Dr. Hillary Lin is a Stanford-trained physician, board-certified internist, and Co-Founder & CEO of CareCore. She hosts The Longevity Show podcast and writes The Longevity Letter. Get weekly longevity insights by subscribing at https://www.hillarylinmd.com/subscribe
.jpg)



.jpg)
.jpg)





